Monday, May 9, 2016
Post 4 Gas Laws
We did the airbag lab today that involved mixing baking soda and vinegar (5%) to produce CO2 in a bag. Starting this lab we found the volume of the bag by filling it with water before converting this amount (mL converted to L) into mols using the equation PV=nRT. After getting the mols, we used this number to find the grams of baking soda needed (stoichiometry) and the mL of vinegar needed (mols of vinegar x 20/1.05 [density]). After that, the lab was smooth sailing.
Sunday, May 8, 2016
Post 3 Gas Laws
Other important laws include:
Charle's Law which involves temperature (in Kelvin) and Volume. It can be used in the equation V1T2 = V2T1.
*remember, when using temperature in Celsius, convert to Kelvin by adding 273.15
chemteam

Avagadro's Law uses volume and moles in a similar equation.
Chemistry
chemteam

Finally, Combined Gas Law is a combination between Charle's Law and Boyle's Law using temp, pressure, and volume.
chemteam

Charle's Law which involves temperature (in Kelvin) and Volume. It can be used in the equation V1T2 = V2T1.
*remember, when using temperature in Celsius, convert to Kelvin by adding 273.15
chemteam

Avagadro's Law uses volume and moles in a similar equation.
Chemistry
chemteam
Finally, Combined Gas Law is a combination between Charle's Law and Boyle's Law using temp, pressure, and volume.
chemteam
Post 2 Gas Laws
Know certain laws that using different parts of the equation (P1V1)/T1 = (P2V2)/T2.
Boyle's Law leaves temp and mols constant while volume and pressure change.
nasa
khanacademy
Common units of pressure:

Boyle's Law leaves temp and mols constant while volume and pressure change.
nasa
khanacademy
Common units of pressure:

Post 1 Gas Laws
Characteristics of Gases:
- gases expand spontaneously to fill their container
- gases are highly compressible
- gases form homogeneous mixtures
- gas molecules are relatively far apart from one another and exert little influence on each other
chem4kids
purdue
And here's some extra details, just for fun.
mikeblaber

- gases expand spontaneously to fill their container
- gases are highly compressible
- gases form homogeneous mixtures
- gas molecules are relatively far apart from one another and exert little influence on each other
chem4kids
purdue
And here's some extra details, just for fun.
mikeblaber
Friday, May 6, 2016
Post 1 Energy and Phase Changes
Energy and phase changes are physical changes of state where intermolecular bonds are broken (not intramolecular bonds).
To determine this, use the equation q=mc∆t where q= thermal energy in J, c= specific heat capacity, and ∆t= change in temperature.


To determine this, use the equation q=mc∆t where q= thermal energy in J, c= specific heat capacity, and ∆t= change in temperature.

Sunday, April 17, 2016
Post 4 Biodiesel
We burned some Chick-Fil-A oil to make our biodiesel fuel for the boats.
Our boat was made from coroplast which is also commonly used in signs and guinea pig cages (which is my personal use for it). We used a whole ton of hot glue to keep the boat water proof but on racing day it passed the line and a little over 22 sec.
Here's some boats in action: youtube
youtube
Our boat was made from coroplast which is also commonly used in signs and guinea pig cages (which is my personal use for it). We used a whole ton of hot glue to keep the boat water proof but on racing day it passed the line and a little over 22 sec.
Here's some boats in action: youtube
youtube
Wednesday, April 6, 2016
Post 3 Biodiesel
Today is a free blog day so I decided to do some extra research on the putt putt boat project. Most noticeable in its appearance in the movie "Ponyo", the putt putt boat is a simple powered, miniture model.
Here's a website that explains how the boat runs and works. sciencetoymaker
And here's a website that explores the differences between multiple fuel sources. consumerreports
Tomorrow we'll be starting the project that involves making a boat and racing it against each other.

Here's a website that explains how the boat runs and works. sciencetoymaker
And here's a website that explores the differences between multiple fuel sources. consumerreports
Tomorrow we'll be starting the project that involves making a boat and racing it against each other.

Tuesday, April 5, 2016
Post 2 Biodiesel
We are now starting a new project on putt putt (pop pop) boats.
Here's a website I found most useful on how to make a putt putt boat. nmia
Here's another website with instructions. allencentre

Here's a website I found most useful on how to make a putt putt boat. nmia
Here's another website with instructions. allencentre
Post 1 Biodiesel
Sunday, March 27, 2016
Post 4 Chemical Bonding
We had a fun pancake party to celebrate the last day of school before spring break!
And here's a site that reviews over the lectures of this unit.
chem1
And here's a site that reviews over the lectures of this unit.
chem1
Sunday, March 13, 2016
Post 3 Chemical Bonding
We did an easy lab that went over chemical bonds by showing us the 3d version of the compound compared to the Lewis structure. As such, it helped us draw diagrams as well as determine the shape created.

Here's an example of our work.

Here's an example of our work.
Saturday, March 12, 2016
Post 2 Chemical Bonding
Resonance is when more than one Lewis structure can be drawn. As such, compounds must have multiple bonds and when you move the bond (double), the neighbor must be able to take the bond like in cases where H can only have one bond and thus can't double bond.
An introduction to this is shown by chemwiki.
Also know the difference in vocab as molecular shape is the molecular geometry (shape) and electronic geometry is determined by the element (lone pairs of electrons and bonds).
Here are some links from khanacademy that go over the unit up to this point.
drawing dot structures
formal charge and dot structures
resonance and dot structures
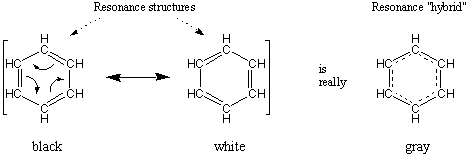
An introduction to this is shown by chemwiki.
Also know the difference in vocab as molecular shape is the molecular geometry (shape) and electronic geometry is determined by the element (lone pairs of electrons and bonds).
Here are some links from khanacademy that go over the unit up to this point.
drawing dot structures
formal charge and dot structures
resonance and dot structures

Post 1 Chemcial Bonding
Our newest lecture is over chemical bonding which involves the type of bonds as well as the shape of those elements as they bond. In covalent bonds, where bonds are shared rather than given or taken, elements can not be between a cation and an anion as this is an ionic bond, and thus this lecture doesn't apply.
First, when given a formula, make a have need share box in which:
H is the number of the element in that bond times the valence number (found in the periodic table).
N comes from the total electrons needed to be stable so you take the number of the element times 7 (where elements become stable but there are exceptions).
S determines how many bonds necessary by taking the added number of needs minus the added number of haves before dividing it by two because all bonds come in pairs of electrons.
The exceptions are:
H, He, Be = 2e-, B = 6e-, and elements in period 3 and beyond can have expanded octets including the major ones, the non-metals S, P, Xe, and Kr.
Here's some links to get into the unit:
hyperphysics

First, when given a formula, make a have need share box in which:
H is the number of the element in that bond times the valence number (found in the periodic table).
N comes from the total electrons needed to be stable so you take the number of the element times 7 (where elements become stable but there are exceptions).
S determines how many bonds necessary by taking the added number of needs minus the added number of haves before dividing it by two because all bonds come in pairs of electrons.
The exceptions are:
H, He, Be = 2e-, B = 6e-, and elements in period 3 and beyond can have expanded octets including the major ones, the non-metals S, P, Xe, and Kr.
Here's some links to get into the unit:
hyperphysics

Wednesday, March 9, 2016
Sunday, March 6, 2016
Post 6 Electronic Structures
We did another worksheet on the periodic table involving finding the different elements and where they would be place on the table. It was a fun but tedious task that involved 42 different elements in which we separated them through metal, metalloids, and non-metals. We then grouped them in columns based off of reaction in which Ah = Na (1+) and Ak = Cl (1-) before ordering them through density. We then fine tuned the chart by looking at ionization, which was the last thing we considered, and trying to keep certain elements we were already aware of in certain areas. In the end, our density across the periods were slightly off. Regardless, you can't help but respect those who have made the period table such an easy chart to maneuver.
Post 5 Electronic Structures
We did a lab involving wavelengths and small vials of liquid to measure the absorbance and %T (transmittance) of the said liquids. In doing so, we have discovered that A and %T have an inverse relationship in which the higher the A, the lower the %T. This corresponds with Beer's law.
Absorbance = e L c in which e is the molar extinction coefficient, L is the path length of the cell holder, and c is the concentration of the solution.

Absorbance = e L c in which e is the molar extinction coefficient, L is the path length of the cell holder, and c is the concentration of the solution.

Thursday, March 3, 2016
Post 4 Electronic Structures
Orbitals:
s - spherical shaped
p - dumb-bell shape with x,y, and z axis
d,f - complex shapes

khanacademy
Rules for Placing Electrons:
Aufbau Principle: electrons enter orbitals of lowest energy first
Pauli Exclusion Principle: an orbital can only contain two electrons with opposite spin
Hund's Rule: within a sublevel, electrons enter singly before pairing up
chemwiki
kentchemistry
s - spherical shaped
p - dumb-bell shape with x,y, and z axis
d,f - complex shapes
khanacademy
Rules for Placing Electrons:
Aufbau Principle: electrons enter orbitals of lowest energy first
Pauli Exclusion Principle: an orbital can only contain two electrons with opposite spin
Hund's Rule: within a sublevel, electrons enter singly before pairing up
chemwiki
kentchemistry
Post 3 Electronic Structures
A brief run down of periodic trends:
electronegativity: increases up, right
electron affinity: increases up, right
ionization energy: increases up, right
atomic radius: increases down, left
nonmetallic character: increases across, up, right
metallic character: increases across, down, left

Here's some websites:
chemwiki
chem
electronegativity: increases up, right
electron affinity: increases up, right
ionization energy: increases up, right
atomic radius: increases down, left
nonmetallic character: increases across, up, right
metallic character: increases across, down, left

Here's some websites:
chemwiki
chem
Post 2 Electronic Structures
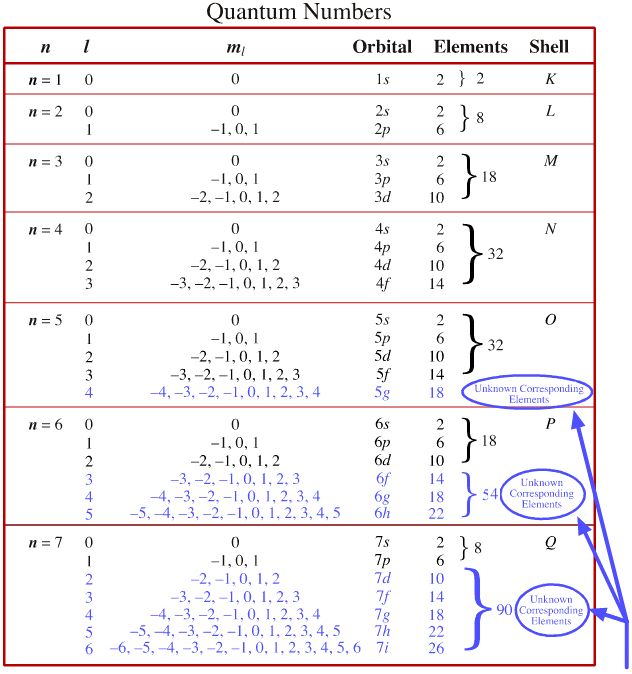
Quantum Numbers:
n - principle quantum number, are generally the periods on the periodic table although they run as s (1-7), p (2-7), d (3-6), and f (4-5)
l - the angular momentum quantum number in which s=0, p=1, d=2, and f=3
m1 - the magnetic quantum number where s is 0, p (-1 to 1), d (-2 to 2), and f runs from -3 to 3
ms - spin quantum number, which represents where the valence electron lands on the orbital (either up or down) in
+1/2 and -1/2 respectively
chemed
n - principle quantum number, are generally the periods on the periodic table although they run as s (1-7), p (2-7), d (3-6), and f (4-5)
l - the angular momentum quantum number in which s=0, p=1, d=2, and f=3
m1 - the magnetic quantum number where s is 0, p (-1 to 1), d (-2 to 2), and f runs from -3 to 3
ms - spin quantum number, which represents where the valence electron lands on the orbital (either up or down) in
+1/2 and -1/2 respectively
chemed
Tuesday, March 1, 2016
Post 1 Electronic Structures
We did the flame test lab that involved testing the color of flames, visible wavelengths, of different metals using a Bunsen burner. The flames were very pretty, ranging from pink to green, and we repeated this process with a glass filter.
We were given a mystery substance, unknown c, which, based on color, is most likely CaCl2. Using the colors, we then determined the wavelengths and used the equation E=h(c/wavelength) to get energy which is then multiplied by Avogadro's number to get J per mol.
Here's a demonstration that goes over a similar process as the lab. youtube
This goes into further detail and provides the wavelengths per color. smc
We were given a mystery substance, unknown c, which, based on color, is most likely CaCl2. Using the colors, we then determined the wavelengths and used the equation E=h(c/wavelength) to get energy which is then multiplied by Avogadro's number to get J per mol.
Here's a demonstration that goes over a similar process as the lab. youtube
This goes into further detail and provides the wavelengths per color. smc
Thursday, February 11, 2016
Post 6 Acid and Base
Today was the test which I found to be very difficult, but regardless here's a review over calculating the Ka from the pH and molarity.
chemwiki
Take the pH and convert this to molarity (H+) by using the equation 10 to the negative x power.
Take this number and square it (x^2), then divide it by the given molarity minus the pH's molarity (x).
chemwiki
Take the pH and convert this to molarity (H+) by using the equation 10 to the negative x power.
Take this number and square it (x^2), then divide it by the given molarity minus the pH's molarity (x).
Wednesday, February 10, 2016
Post 5 Acid and Base
Post 4 Acid and Base
We finished a second lab that involved a similar run as the first but we now had an unknown solid acid that we used. First we had to dissolve the solid in the water using heat before cooling it down and titrating it.
Here we placed it on a plate burner with a stirring rod.
Here it turns vibrant pink but the color begins to fade after awhile.
Here we placed it on a plate burner with a stirring rod.
Here it turns vibrant pink but the color begins to fade after awhile.
Sunday, February 7, 2016
Post 3 Acid and Base
We did another vinegar lab that involved titrating KHP and vinegar samples using NaOH that turned the solution bright pink after mixing with phenolphthalein. It was the first time we used a buret, and it was difficult to control the drops, but regardless, it was a fun lab.
Here's the procedure:
Here's some websites that go over it.
khanacademy
chemwiki
Here's the procedure:
1) Put on your splash-proof safety
glasses, and keep them on throughout the lab.
2) Drain your buret into the waste beaker.
3) Rinse your buret with a small amount (several mL) of your NaOH
solution, and drain into your waste beaker.
4) With the valve closed, add your NaOH solution until it is above
the zero mark, and carefully drain until the meniscus is exactly at
zero.
5) Obtain 0.5 – 0.8 gram of KHP, and record the mass to the
nearest 0.001 g.
6) Transfer the KHP (without losing any) to the Erlenmeyer flask.
Rinse the plastic tray with several mL of distilled water, and pour
the rinse water into the Erlenmeyer. The goal is to transfer 100% of
the KHP into the flask.
7) Add about 75 mL of water to the Erlenmeyer, and swirl to dissolve
all of the KHP.
8) Add 2-3 drops of phenolphthalein.
9) Titrate the sample until a faint pink color remains, and record
the volume of NaOH used to the nearest 0.1 mL.
10) Open up the vial with the blue cap. This vial contains
commercial vinegar.
11) Pipet 10.00 mL of the vinegar into the Erlenmeyer flask. Dilute
the volume to about 100 mL with distilled water, add two drops of
phenolphthalein, and titrate with your NaOH solution. Record the
volume of NaOH used.
12) If time permits, repeat steps 5-11.
13) Drain your buret, fill it with distilled water, and clean up you
station.
khanacademy
chemwiki
Sunday, January 31, 2016
Post 2 Acid and Base
Getting for the first quiz of this unit.
Acids [H+] and bases [OH-] together produce a salt and water. Acids produce conjugate bases and bases produces conjugate acids. These can then change through reactions.
When converting H+ to OH- use the equation H+ = Kw/ OH- in which Kw = 1.0 x 10^-14 and this equation can be used with OH- interchangeably
H+ to pH and OH- to pOH uses -log and going from pH to pOH would subtract 14.
Here's a link that provides information. mrwiggersci

Acids [H+] and bases [OH-] together produce a salt and water. Acids produce conjugate bases and bases produces conjugate acids. These can then change through reactions.
When converting H+ to OH- use the equation H+ = Kw/ OH- in which Kw = 1.0 x 10^-14 and this equation can be used with OH- interchangeably
H+ to pH and OH- to pOH uses -log and going from pH to pOH would subtract 14.
Here's a link that provides information. mrwiggersci
Wednesday, January 27, 2016
Post 1 Acid and Base
We did another lab that involved the titration of vitamin c and iodine, which produced a clear color, and starch, which turned the solution blue to determine the concentration of vitamin c within different juices. Unsurprisingly, the grapefruit juice contained the most.
Sunday, January 17, 2016
Post 3 Aqueous Solutions
Our latest unit includes both stoichiometry and molarity in calculations that, just like stoichiometry, convert volume (ml, L) using molarity (mol/L) into mols which are then converted into the new unit.
Here's a video that goes over it. youtube
Here's another website that goes over the step by step process of calculating molarity using stoichiometry. yeahchemistry
Here's a video that goes over it. youtube
Here's another website that goes over the step by step process of calculating molarity using stoichiometry. yeahchemistry
Thursday, January 14, 2016
Post 2 Aqueous Solutions
We have recently finished gathering the data for the murder investigation lab which involved double replacement chemical reactions, determining which reactions produced solids which when then used to determine that the mystery poison was silver carbonate. Here's a link to the lab. murder mystery lab
We discovered that the culprit was Mr.Green.
Here's a reminder on: double replacement youtube
solubility rules chem
molarity occc , chemteam
We discovered that the culprit was Mr.Green.
Here's a reminder on: double replacement youtube
solubility rules chem
molarity occc , chemteam
Sunday, January 10, 2016
Post 1 Aqueous Solutions
We did a lab that went over the concentration of stocks which involved diluting a stock of food colored water until the coloring had disappeared. Overall, it was a fun simple lab that got us into the new unit.
Here's a website that goes over it. chemteam
Here's a website that goes over it. chemteam
Subscribe to:
Comments (Atom)