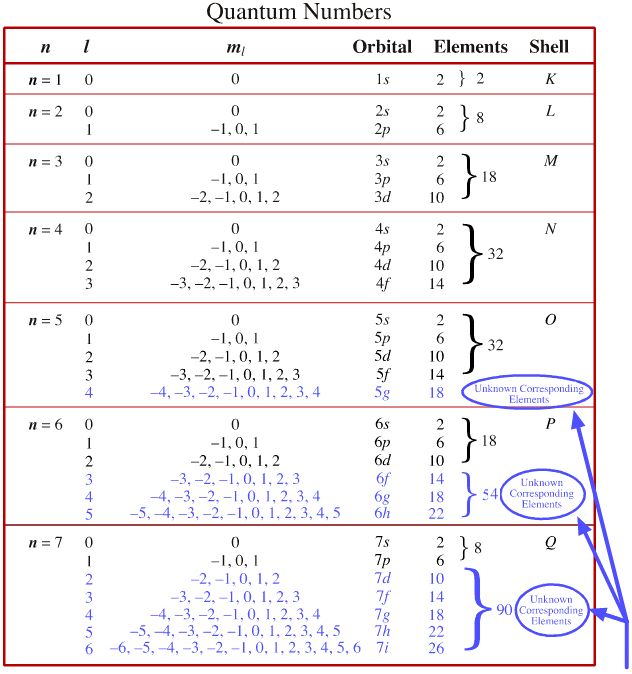
Quantum Numbers:
n - principle quantum number, are generally the periods on the periodic table although they run as s (1-7), p (2-7), d (3-6), and f (4-5)
l - the angular momentum quantum number in which s=0, p=1, d=2, and f=3
m1 - the magnetic quantum number where s is 0, p (-1 to 1), d (-2 to 2), and f runs from -3 to 3
ms - spin quantum number, which represents where the valence electron lands on the orbital (either up or down) in
+1/2 and -1/2 respectively
chemed
No comments:
Post a Comment