An introduction to this is shown by chemwiki.
Also know the difference in vocab as molecular shape is the molecular geometry (shape) and electronic geometry is determined by the element (lone pairs of electrons and bonds).
Here are some links from khanacademy that go over the unit up to this point.
drawing dot structures
formal charge and dot structures
resonance and dot structures
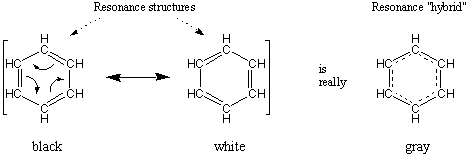
Emmeline, I thought your post was very educational. I like how you first established what resonance is and explained it with an example, showing you fully understood the concept. In addition, you clearly differentiated between molecular shape and electronic geometry, as they can be easily confused. Lastly, I found the first website you left a link to very helpful, describing formal charge and resonance hybrids.
ReplyDelete