Day 3 of the lab.
Today we finished weighing the mass of the jar and copper and calculated the % yield to determine the charge of the Iron. First we subtracted the original mass to the new mass of the nail after the reaction and did the same with the copper (jar+copper-jar mass) to get how much copper was left. We then converted the mass of the iron into copper to get the theoretical yield which was over 100%, if the iron was a 2+ charge, and a little over 70% if calculated at 3+ leading us to believe that the Fe was a 3+.
Here's some more information on finding the theoretical yield. Tripod
Monday, December 14, 2015
Sunday, December 13, 2015
Post 3 Stoichiometry
Day 2 of the lab.
After the nail has reacted over night, we pulled it out and proceeded to wash and rinse the whole jar while making sure to avoid losing any copper through the process as this will be needed to determine if our element has a 2+ or 3+ charge. We are now allowing the remainder of the liquid to fully dry before we begin weighing the copper.
After the nail has reacted over night, we pulled it out and proceeded to wash and rinse the whole jar while making sure to avoid losing any copper through the process as this will be needed to determine if our element has a 2+ or 3+ charge. We are now allowing the remainder of the liquid to fully dry before we begin weighing the copper.
Saturday, December 12, 2015
Post 2 Stoichiometry
Day 1 of the lab.
Today we placed 4 grams of copper II chloride, CuCl2, into a baby food jar, added 50mL of water and stirred. The water became a bright, pretty blue. We then, after polishing, placed an iron nail inside which immediately began collecting the copper into a rusted red substance.
Here's a worksheet that further explains the lab. quia
Today we placed 4 grams of copper II chloride, CuCl2, into a baby food jar, added 50mL of water and stirred. The water became a bright, pretty blue. We then, after polishing, placed an iron nail inside which immediately began collecting the copper into a rusted red substance.
Here's a worksheet that further explains the lab. quia
Wednesday, December 9, 2015
Post 1 Stoichiometry
Stoichiometry is our next unity that involves finding the relationships, measurements, between reactants and products within a chemical reaction. It begins with balancing the equation, finding the molar mass of the reactant then converted to mols, using the coefficients (mols) of the reactant to the converted product, and then converting the mols of the new product back to mass (grams).

Using this same method, you can use stoichiometry to find the limiting reagent within a reaction by calculating each of the reactants using the same conversion method as described above, converting it into the product, and then finding which produces the least amount of product using the given mass. This is the LR which can only produce so much of the product leaving the other reactant with excess.

When determining the excess reactants left over, use the amount of product created with the limiting reactant and convert it using the same formula as stated before into the excess reactant to find how much of that element (species) was used up in the chemical reaction. Then subtract this answer from the original mass of that reactant that you began with to find how much was not used in the reaction (or left over). This is your excess.

Finally, stoichiometry is also used with the chemical equation Yield% = 100X(actual yield/theoretical yield)
When doing all of these formulas, make sure the equation is balanced and the only transition of elements can be calculated through mols.
Here's some sites that may help.
khanacademy
CrashCourse
chemwiki
chemteam

Using this same method, you can use stoichiometry to find the limiting reagent within a reaction by calculating each of the reactants using the same conversion method as described above, converting it into the product, and then finding which produces the least amount of product using the given mass. This is the LR which can only produce so much of the product leaving the other reactant with excess.

When determining the excess reactants left over, use the amount of product created with the limiting reactant and convert it using the same formula as stated before into the excess reactant to find how much of that element (species) was used up in the chemical reaction. Then subtract this answer from the original mass of that reactant that you began with to find how much was not used in the reaction (or left over). This is your excess.

Finally, stoichiometry is also used with the chemical equation Yield% = 100X(actual yield/theoretical yield)
When doing all of these formulas, make sure the equation is balanced and the only transition of elements can be calculated through mols.
Here's some sites that may help.
khanacademy
CrashCourse
chemwiki
chemteam
Wednesday, December 2, 2015
Post 5 Chemical Reactions
Yesterday we did the lab which included single replacement reactions by combining chemicals and elements. Some of the reactions resulted in smoking, fizzing, and change in coloration which was all interesting to see.
Friday, November 27, 2015
Post 4 Chemical Reactions
In transfer of electrons, or RedOx, is electrons being transferred from the metal to the non-metal.
 There are five main types of RedOx reactions that are further explained in this website although they call synthesis combination.
There are five main types of RedOx reactions that are further explained in this website although they call synthesis combination.
boundless
Also, the element being oxidized, losing electrons, is the reducing agent while the element being reduced, gaining electrons, is the oxidizing agent. When giving those agents, look at the reactants, not the products.
Furthermore, during single replacement, for an element to "attack" or replace another in the compound, it must be higher on the Acid Strength Table (Reactivity Series for Metals) hence why Li can replace anyone and Au can not.
boundless
Also, the element being oxidized, losing electrons, is the reducing agent while the element being reduced, gaining electrons, is the oxidizing agent. When giving those agents, look at the reactants, not the products.
Furthermore, during single replacement, for an element to "attack" or replace another in the compound, it must be higher on the Acid Strength Table (Reactivity Series for Metals) hence why Li can replace anyone and Au can not.
Thursday, November 26, 2015
Post 3 Chemical Reactions
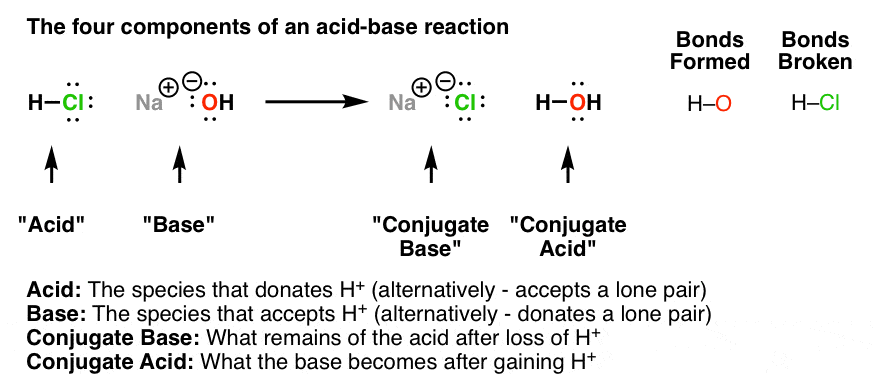
Acid/Base Reactions: produce water and a 'salt' and salt is a cation of a base and anion of an acid.
To tell a strong acid, typically see if the O outnumbers the H by two or more. Another way is if there is only one H and no oxygen in cases of the diatomic elements (BrICl).
Here's a website that goes in to further detail. chemguide
To tell a strong base, they contain -OH anions and you can find more here. chemguide
Also here's a website over acid base reactions. crashcourse
When you're tired of studying, here's a great video to cheer you up. acapellascience2
To tell a strong acid, typically see if the O outnumbers the H by two or more. Another way is if there is only one H and no oxygen in cases of the diatomic elements (BrICl).
Here's a website that goes in to further detail. chemguide
To tell a strong base, they contain -OH anions and you can find more here. chemguide
Also here's a website over acid base reactions. crashcourse
When you're tired of studying, here's a great video to cheer you up. acapellascience2

Saturday, November 21, 2015
Post 2 Chemical Reactions
We have now been learning on balancing and writing the products of the reactants which, unlike the previous method, requires two ionic aqueous compounds that switch elements in the product. You must then determine if the resultant is aqueous (spectator ions) or solid (the reactants) using the solubility rules. Here's a link that provides interesting ways to remember these rules. youtube
And here's another video to go over the equations along with other review. CrashCourse

And here's another video to go over the equations along with other review. CrashCourse

Wednesday, November 18, 2015
Post 1 Chemical Reactions
We just started a new unit beginning with the CHO method and combustion of balancing equations.
Combustion: 1. will always react with oxygen,
2. is an exothermic reaction
3. always produces CO2 and H2O
We began are unit with just balancing equations. If the prefix is represented in a decimal, the whole equation should be multiplied to equal whole numbers. Also, don't forget that, opposed to the common nomenclature rules, methane is CH4 and ammonia is NH3.
Here's a good video to start the unit off. khanacademy

Combustion: 1. will always react with oxygen,
2. is an exothermic reaction
3. always produces CO2 and H2O
We began are unit with just balancing equations. If the prefix is represented in a decimal, the whole equation should be multiplied to equal whole numbers. Also, don't forget that, opposed to the common nomenclature rules, methane is CH4 and ammonia is NH3.
Here's a good video to start the unit off. khanacademy

Sunday, November 15, 2015
Post 4 Chemical Composition
Monday is the test and I have been studying mostly significant digits, converting moles, and units. Don't forget the diatomics HOFBrINCl which is doubled the mass when alone. Here's a good review for converting moles.
khanacademy

khanacademy

Thursday, November 12, 2015
Post 3 Chemical Composition
Today was the Formula of a Chloride Lab which included heating a mixture of Zinc and HCL to get a compound in which you had to determine the formula using the data found. Using the given data, my partner and I determined the formula to be ZnCl2. Here's a few websites that can help.
chemteam
tamu
about
youtube

chemteam
tamu
about
youtube
Sunday, November 8, 2015
Post 2 Chemical Composition
So we are now learning about hydrate compounds and anhydrates. The nomenclature of hydrates stays relatively the same, but don't forget to name the number of water molecules (type III prefixes) before the hydrate/hydride. To calculate this number convert both the mass of the crucible and the mass of the hydrate to moles using the amount in grams divided by the total mass of the molecules/compound. You then divide the large number (moles of H20) by the smaller (moles of anhydrous salt) to get x (value of hydrate).
Here's a reminder over the nomenclature. purdue
And here's a practice of my worksheet.
Here's a reminder over the nomenclature. purdue
And here's a practice of my worksheet.
Wednesday, November 4, 2015
Post 1 Chemical Composition
Yesterday we took the pretest which was very difficult and worries me for the future. Today we learned about converting units using mole, 6.02 × 10 23, and the mole road map.
Here's a website that helps explain converting grams to moles.
ChemTeam

Here's a website that helps explain converting grams to moles.
ChemTeam
Wednesday, October 28, 2015
Post 5 Measurements
Tomorrow will be the quiz over measurements and converting units. Here's a list of most commonly used units of measurements and conversions.
conweb
conweb
Tuesday, October 20, 2015
Post 4 Measurements
Today we did the pre-test for the new unit which mostly consisted of math and converting measurements. Here's a video that goes over the same concepts.
CrashCourse
CrashCourse
Tuesday, October 13, 2015
Post 3 Measurements
Here are some websites that can help convert measurements.
sciencemadesimple
asknumbers
I also use this website when trying to find new recipes.
allrecipes
sciencemadesimple
asknumbers
I also use this website when trying to find new recipes.
allrecipes
Friday, October 9, 2015
Post 2 Measurements
Over fall break, we must calculate the measurements of three recipes using the metric system. Here is a helpful site.
mhhe
mhhe
Tuesday, October 6, 2015
Post 1 Measurements
We started the aspirin lab yesterday and finished up today. Using salicylic acid mixed with acetic anhydride, then boiled in a hot water bath before condensing into crystals that are later rinsed through ice cold water before being drained and left to dry on day 2. My partner and I had not dissolved the acids properly before boiling resulting in the salicylic acid burning and turning an icky green resulting in yellow crystals. Regardless, it was a fun lab. Now we are just waiting for the results after fall break.
Thursday, October 1, 2015
Post 7 Atomic Structure and Radioactivity
Today we did the radioactivity quiz over half-life, radioactive decay, and reviewing atoms and nomenclature. Here is a video link with a general review over half-life and radioactive decay.
Crash Course
Crash Course
Wednesday, September 30, 2015
Post 6 Atomic Structure and Radioactivity
Monday, September 28, 2015
Post 5 Atomic Structure and Radioactivity
We learned about the half lives of radioactive elements today and played a game consisting of cutting out .5in squares, shaking the squares in a cup and removing the turned squares, represented as one half life, before repeating the process. I also found a good website for the star blog to help with light spectrum of elements and what elements are found in specific stars.
Visible Spectra of the Elements
Stellar Database
Lunapic (used for cropping pictures of visible spectrum)
Visible Spectra of the Elements
Stellar Database
Lunapic (used for cropping pictures of visible spectrum)
Tuesday, September 22, 2015
Post 4 Atomic Structure and Radioactivity
We did the Atomic Structure and Radioactivity lab today. My partner and I counted beans (atoms), found the mass of the beans totals, divided the mass by the amount to get the avg. mass of each bean (isotopes), found the % abundance of each isotope by dividing number of atoms by total number of beanuim atoms (beans in total), and then we calculated the average atomic mass through the equation ((mass)(%)) + ((mass)(%)) and got 0.280885 as the average.

Monday, September 21, 2015
Post 3 Atomic Structure and Radioactivity
Today we learned about how to find the number of protons (the atomic number), neutrons (atomic mass-atomic number), and the electron (depending on if it is an isotope, the electrons will be equal to the number of protons). To find the atomic mass, you must use the equation ((mass)(%))+ ((mass)(%)).

Subscribe to:
Comments (Atom)








