To tell a strong acid, typically see if the O outnumbers the H by two or more. Another way is if there is only one H and no oxygen in cases of the diatomic elements (BrICl).
Here's a website that goes in to further detail. chemguide
To tell a strong base, they contain -OH anions and you can find more here. chemguide
Also here's a website over acid base reactions. crashcourse
When you're tired of studying, here's a great video to cheer you up. acapellascience2
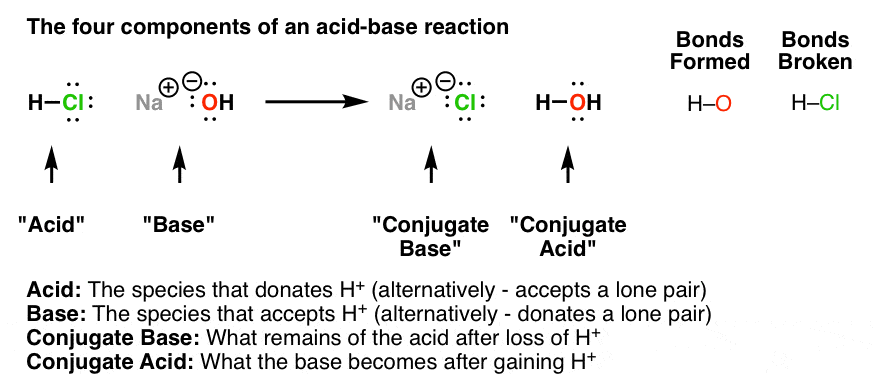
This post contains a lot of useful information. I liked how you included descriptions, an image, and multiple links for further reference. Thanks so much for posting.
ReplyDelete