We had a fun pancake party to celebrate the last day of school before spring break!
And here's a site that reviews over the lectures of this unit.
chem1
Sunday, March 27, 2016
Sunday, March 13, 2016
Post 3 Chemical Bonding
We did an easy lab that went over chemical bonds by showing us the 3d version of the compound compared to the Lewis structure. As such, it helped us draw diagrams as well as determine the shape created.

Here's an example of our work.

Here's an example of our work.
Saturday, March 12, 2016
Post 2 Chemical Bonding
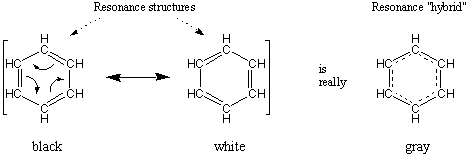
Resonance is when more than one Lewis structure can be drawn. As such, compounds must have multiple bonds and when you move the bond (double), the neighbor must be able to take the bond like in cases where H can only have one bond and thus can't double bond.
An introduction to this is shown by chemwiki.
Also know the difference in vocab as molecular shape is the molecular geometry (shape) and electronic geometry is determined by the element (lone pairs of electrons and bonds).
Here are some links from khanacademy that go over the unit up to this point.
drawing dot structures
formal charge and dot structures
resonance and dot structures
An introduction to this is shown by chemwiki.
Also know the difference in vocab as molecular shape is the molecular geometry (shape) and electronic geometry is determined by the element (lone pairs of electrons and bonds).
Here are some links from khanacademy that go over the unit up to this point.
drawing dot structures
formal charge and dot structures
resonance and dot structures

Post 1 Chemcial Bonding
Our newest lecture is over chemical bonding which involves the type of bonds as well as the shape of those elements as they bond. In covalent bonds, where bonds are shared rather than given or taken, elements can not be between a cation and an anion as this is an ionic bond, and thus this lecture doesn't apply.
First, when given a formula, make a have need share box in which:
H is the number of the element in that bond times the valence number (found in the periodic table).
N comes from the total electrons needed to be stable so you take the number of the element times 7 (where elements become stable but there are exceptions).
S determines how many bonds necessary by taking the added number of needs minus the added number of haves before dividing it by two because all bonds come in pairs of electrons.
The exceptions are:
H, He, Be = 2e-, B = 6e-, and elements in period 3 and beyond can have expanded octets including the major ones, the non-metals S, P, Xe, and Kr.
Here's some links to get into the unit:
hyperphysics

First, when given a formula, make a have need share box in which:
H is the number of the element in that bond times the valence number (found in the periodic table).
N comes from the total electrons needed to be stable so you take the number of the element times 7 (where elements become stable but there are exceptions).
S determines how many bonds necessary by taking the added number of needs minus the added number of haves before dividing it by two because all bonds come in pairs of electrons.
The exceptions are:
H, He, Be = 2e-, B = 6e-, and elements in period 3 and beyond can have expanded octets including the major ones, the non-metals S, P, Xe, and Kr.
Here's some links to get into the unit:
hyperphysics

Wednesday, March 9, 2016
Sunday, March 6, 2016
Post 6 Electronic Structures
We did another worksheet on the periodic table involving finding the different elements and where they would be place on the table. It was a fun but tedious task that involved 42 different elements in which we separated them through metal, metalloids, and non-metals. We then grouped them in columns based off of reaction in which Ah = Na (1+) and Ak = Cl (1-) before ordering them through density. We then fine tuned the chart by looking at ionization, which was the last thing we considered, and trying to keep certain elements we were already aware of in certain areas. In the end, our density across the periods were slightly off. Regardless, you can't help but respect those who have made the period table such an easy chart to maneuver.
Post 5 Electronic Structures
We did a lab involving wavelengths and small vials of liquid to measure the absorbance and %T (transmittance) of the said liquids. In doing so, we have discovered that A and %T have an inverse relationship in which the higher the A, the lower the %T. This corresponds with Beer's law.
Absorbance = e L c in which e is the molar extinction coefficient, L is the path length of the cell holder, and c is the concentration of the solution.

Absorbance = e L c in which e is the molar extinction coefficient, L is the path length of the cell holder, and c is the concentration of the solution.

Thursday, March 3, 2016
Post 4 Electronic Structures
Orbitals:
s - spherical shaped
p - dumb-bell shape with x,y, and z axis
d,f - complex shapes

khanacademy
Rules for Placing Electrons:
Aufbau Principle: electrons enter orbitals of lowest energy first
Pauli Exclusion Principle: an orbital can only contain two electrons with opposite spin
Hund's Rule: within a sublevel, electrons enter singly before pairing up
chemwiki
kentchemistry
s - spherical shaped
p - dumb-bell shape with x,y, and z axis
d,f - complex shapes
khanacademy
Rules for Placing Electrons:
Aufbau Principle: electrons enter orbitals of lowest energy first
Pauli Exclusion Principle: an orbital can only contain two electrons with opposite spin
Hund's Rule: within a sublevel, electrons enter singly before pairing up
chemwiki
kentchemistry
Post 3 Electronic Structures
A brief run down of periodic trends:
electronegativity: increases up, right
electron affinity: increases up, right
ionization energy: increases up, right
atomic radius: increases down, left
nonmetallic character: increases across, up, right
metallic character: increases across, down, left

Here's some websites:
chemwiki
chem
electronegativity: increases up, right
electron affinity: increases up, right
ionization energy: increases up, right
atomic radius: increases down, left
nonmetallic character: increases across, up, right
metallic character: increases across, down, left

Here's some websites:
chemwiki
chem
Post 2 Electronic Structures
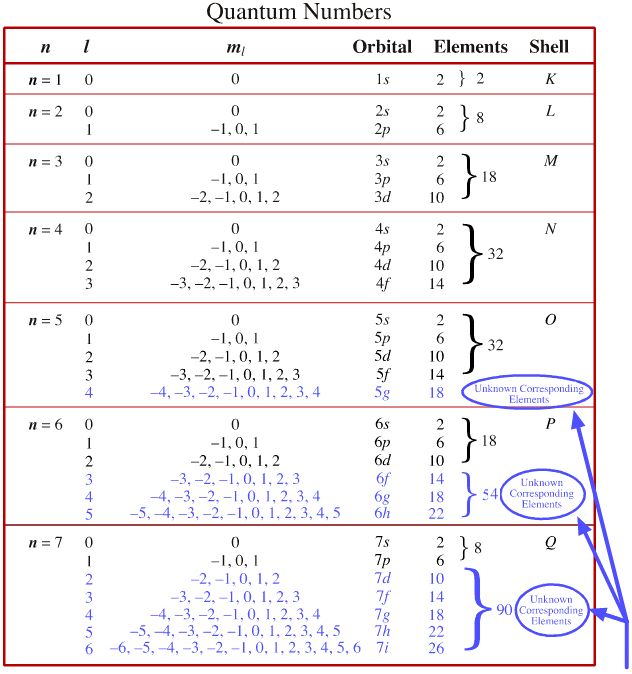
Quantum Numbers:
n - principle quantum number, are generally the periods on the periodic table although they run as s (1-7), p (2-7), d (3-6), and f (4-5)
l - the angular momentum quantum number in which s=0, p=1, d=2, and f=3
m1 - the magnetic quantum number where s is 0, p (-1 to 1), d (-2 to 2), and f runs from -3 to 3
ms - spin quantum number, which represents where the valence electron lands on the orbital (either up or down) in
+1/2 and -1/2 respectively
chemed
n - principle quantum number, are generally the periods on the periodic table although they run as s (1-7), p (2-7), d (3-6), and f (4-5)
l - the angular momentum quantum number in which s=0, p=1, d=2, and f=3
m1 - the magnetic quantum number where s is 0, p (-1 to 1), d (-2 to 2), and f runs from -3 to 3
ms - spin quantum number, which represents where the valence electron lands on the orbital (either up or down) in
+1/2 and -1/2 respectively
chemed
Tuesday, March 1, 2016
Post 1 Electronic Structures
We did the flame test lab that involved testing the color of flames, visible wavelengths, of different metals using a Bunsen burner. The flames were very pretty, ranging from pink to green, and we repeated this process with a glass filter.
We were given a mystery substance, unknown c, which, based on color, is most likely CaCl2. Using the colors, we then determined the wavelengths and used the equation E=h(c/wavelength) to get energy which is then multiplied by Avogadro's number to get J per mol.
Here's a demonstration that goes over a similar process as the lab. youtube
This goes into further detail and provides the wavelengths per color. smc
We were given a mystery substance, unknown c, which, based on color, is most likely CaCl2. Using the colors, we then determined the wavelengths and used the equation E=h(c/wavelength) to get energy which is then multiplied by Avogadro's number to get J per mol.
Here's a demonstration that goes over a similar process as the lab. youtube
This goes into further detail and provides the wavelengths per color. smc
Subscribe to:
Comments (Atom)