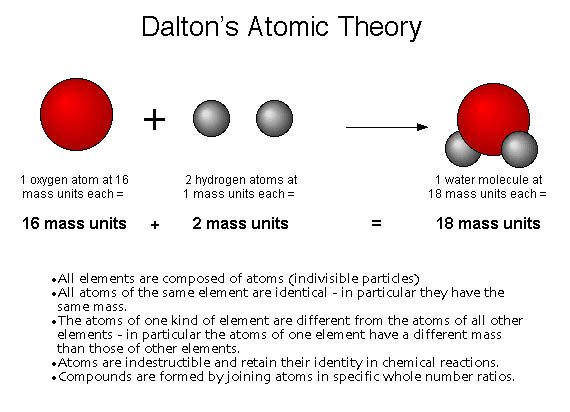
Today, in class, we learned about Dalton's Atomic Theory, Law of Constant Composition, how to calculate the percent of compositions, and how atomic models have evolved over time thanks to the influence of Thomson, who discovered electrons, and Rutherford, who discovered protons.